Original Research Article - Journal of Apitherapy (2022)
Lebanese Propolis from Different Regions: Phytochemical Screening, Antioxidant Activity and Effect against Cancer Cells
Doha Kabani1,2, Ali Jaber1*, Fadi Abdel Sater2, Ghassan Ibrahim1 and Edmond Cheble12Department of Sciences, Lebanese University, Hadath-Beirut, Lebanon
Ali Jaber, Department of Pharmacy, Lebanese University, Beirut, Lebanon, Tel: 9613451884, Email: ali.jaber.2@ul.edu.lb
Received: 31-Jan-2022, Manuscript No. JAPITHERAPY-22-52876; Editor assigned: 02-Feb-2022, Pre QC No. JAPITHERAPY-22-52876 (PQ); Reviewed: 17-Feb-2022, QC No. JAPITHERAPY-22-52876; Revised: 22-Feb-2022, Manuscript No. JAPITHERAPY-22-52876 (R); Published: 01-Mar-2022
Abstract
Aim: Apitherapy, preparations, and food and beverage additives all employ propolis. Chemical content, biological activities as well, highly depend on the origins of propolis. This study aimed to compare the Total phenolic contents, antioxidant activity, and cytotoxicity to cancer cell lines of Lebanese propolis extracts from four regions.
Materials and methods: The methanolic extracts of propolis collected were prepared in the first step. The samples were subjected to phytochemical screening for the first time. In a second time, samples were tested for total phenol content by the Folin–Ciocalteau method, and radical scavenging activity using a spectrophotometric method. Anticancer activity was assayed on human tumor cell lines MDA-MB-231 and A549, using MTT assay.
Results: The results show that the estimation of the total phenolics varies between 3.60 mg and 73.52 mg of GAE / g of propolis extract and / g of propolis extract. The inhibition concentration values of 1, 1-Diphenyl-2-picrylhydrazyl radical scavenging and ascorbic acid in propolis in Fakeha, Debaal, Wadi Faara, and Qlaileh were 25.9 ± 5, 26.3 ± 4, 57.1 ± 10 and 729.7 ± 42 (μg.mL−1), respectively. Additionally, propolis extracts 1 mg.mL-1 from two regions, namely Wadi Faara (31.71%) and Debaal (23.3%) demonstrated the inhibitory effect on proliferation of A549 cancer cell lines at 72 hours in a dose-dependent manner. However, these extracts show the opposite effect on breast cancer cells.
Conclusion: These results are of interest since Lebanese propolis from some regions has antioxidant properties and decreases the percentage of cell viability of human tumor cells A549; thus, it has the potential to contain some chemical compounds acting as an anticancer drug.
Keywords
Propolis; secondary metabolites; phenolic compounds; antioxidant activity; anticancer activity
Introduction
Propolis is a natural resinous product assembled by honey bees (Apis mellifera) from different plant sources. It’s used to make the protective shield at the entrance of beehives and it was already known for its medicinal powers for generations when the product was employed in embalming bodies in Egypt [1]. Nowadays, propolis piqued scientists’ interest due to its broad range of activities that can be used in complementary therapies, based on the biological and pharmacological properties that have been demonstrated including antitumor, antibacterial, antioxidant, antifungal, and other activities [2-5].
Propolis is generally composed of 50% resin and balm (including phenolic compounds), 30% wax and fatty acids, 10% essential oils, 5% pollen, and 5% various organic and inorganic compounds. The specific composition of propolis depends on the vegetation at the site of collection [6].
Among all the bee products, propolis possesses the highest antioxidant activity [7]. The antioxidant potential of propolis was originated from their polyphenolic substances [8,9]. Thus, propolis can be used for the prevention and treatment of diseases related to the increase of oxidative stress such as cancer, aging, and cardiovascular diseases [10].
On the other hand, cancer has become one of the major diseases and problems that have caused predominant death, and it is considered the second cause of death after cardiovascular diseases [11]. The typical cancer treatment is generally based on using chemotherapy, radiotherapy, cytotoxic drugs, and surgery [12,13]. These conventional therapies are effective and can even cure many types of cancers including breast cancer, colon, pancreatic, testicular, ovarian, and certain lung cancers, but their effectiveness is often limited by toxic effects [14]. Thus, continued searching for a safer and more effective treatment is needed [15]. For many years’ natural medicines have been used and are still used in developing countries as the primary source of medical treatment [16]. Among many other potential anti-cancer natural sources propolis was demonstrated to have different anti-cancer effects going from selectively cytotoxic, anti-proliferative, and pro-apoptotic against tumor cells, to anti-metastatic, anti-mutagenic, anti-invasive, and anti-angiogenic [17– 20].
Propolis’s physical, chemical, and biological properties vary according to its botanical and geographical origins, which lead to variation in the chemical composition [21– 23]. Therefore, several types of propolis are known based on the geographical area of the hive, the plants present in this geographical area, the availability of the plants during the season, and the species of bees [24].
This work comes in the continuation of an investigation aiming to study the Lebanese propolis, and the effect of biological environments on the quality of the propolis produced. In the present study, methanolic propolis extracts from four different Lebanese locations were prepared. Thus, the effect of geographical origin on the phytochemical contents was assessed and results were compared for the antioxidant capacity in terms of free radical scavenging assay and reducing power assay.
Materials and Methods
Chemicals and instrumentation
All chemicals used were of analytical grade. Methanol (MeOH), Ethanol (EtOH), chloroform, Folin Ciocalteu, and ascorbic acid were purchased from BDH (England). 2, 2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, sodium carbonates anhydrous (Na2CO3), potassium ferricyanide, trichloroacetic acid, and iron (III) chloride (FeCl3), were purchased from Sigma Aldrich (USA). Samples were weighed using a RADWAG XA 82/220/2X laboratory balance. The absorbance values were measured using a VWR UV-6300PC double beam spectrophotometer and extracts were concentrated using HEIDOLPH (Germany) rotavapor apparatus.
Samples collection
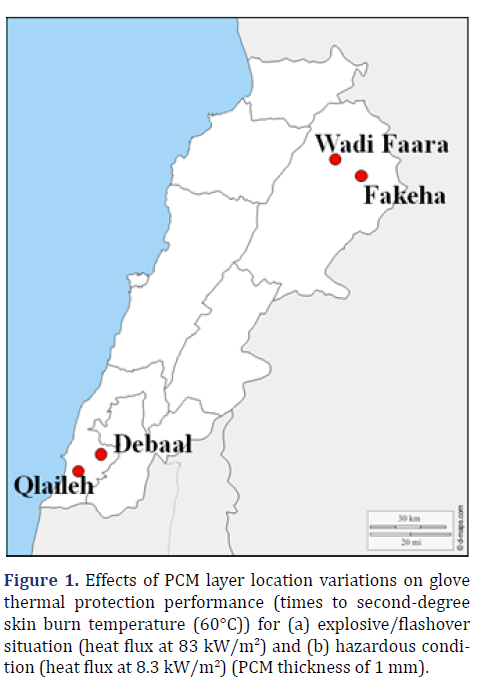
In this study, four Lebanese Apis mellifera propolis samples were collected (April 2019) from four apiaries located in different Lebanese regions (Figure 1), more specifically from Debaal (33°15′02″N, 35°20′56″E), Fakeha (34°14′44″N, 36°24′21″E), Qlaileh (33°19′87″N, 35°22′91″E) and Wadi-Faara (34°17’22.0” N 36°18’15.8” E). The main process to collect the raw propolis samples was started by the initial preparation to separate it from extraneous macro impurities if present. The obtained samples were frozen at -20˚C until analysis.
Preparation of methanolic extract of propolis (MEP)
The methanolic extract of propolis (MEP) was obtained according to the procedure described by Li, et al. [25] with slight modifications. Briefly, one gram of each frozen brown to yellow propolis sample was chopped into small pieces and immediately homogenously pulverized. Then, each sample was sonicated in 20 mL of MeOH for 2 hours using an ultrasonic bath at 25°C. The mixture was then filtered through Whatman filter No.1, and the filtrate was evaporated under reduced pressure to produce the methanolic extract of propolis (MEP). Finally, the MEP was weighed and stored at 4°C for further use.
Determination of percentage yield (%)
The extraction yield was calculated according to the following equation (1):
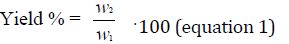
Where W1 is the dry weight of the used matter and W2 is the weight of collected extract after evaporation of the solvent.
Phytochemical screening
The qualitative phytochemistry tests of the MEP samples were carried out through phytochemical characterization tests via coloring or precipitation reactions on the ex-tracts [26,27].
Test for alkaloids
About 2 mL of extracts were treated with Dragendorff’s reagent (solution of Potassium Bismuth Iodide). The formation of a red precipitate indicates the presence of alkaloids.
Anthraquinones (Borntrager’s reaction for free anthraquinone)
In a dry test tube, 3 mL of MEPs were mixed with 10 mL of chloroform. This was steamed for 5 minutes in a steam bath and directly filtered before cooling. An equivalent volume of a 10% ammonia solution was added to the filtrate. This was shaken, and brilliant pink coloration in the upper aqueous layer was noticed, indicating the presence of Anthraquinones.
Test for terpenoids (Salkowski test)
1 mL of each extract was mixed in 2 mL of chloroform, and concentrated H2SO4 (3 mL) was carefully added to form a layer. A reddish brown coloration of the interface was formed to show positive results for the presence of Terpenoids [28].
Test for hydrolysable tannins
A few drops of 0.1% ferric chloride were added and observed for brownish green or a blue-black coloration.
Test for quinones
1 mL of concentrated hydrochloric acid (HCl) was added to one mL of MEP. The presence of quinones is confirmed by the appearance of yellow color.
Test for flavonoids
1 mL of KOH is added to 1 mL of each extract. The yellow shift shows the existence of flavonoids.
Test for Saponins (Frothing Test)
1 mL of extract was shaken with 2 mL of water. If foam produced persists for ten minutes it indicates the presence of saponins.
Determination of total phenolic content (TPC)
The total polyphenol content in propolis extract was determined by using the Folin-Ciocalteu method [29] with some modifications. Briefly, 20 μL of the MEP extract was taken and mixed with 1500 μL distilled water, then 100 μL of aqueous Folin-Ciocalteu solution. After 5 min incubation at room temperature, 300 μL of sodium carbonate (7.5%) were added. The obtained mixture was allowed to stand for 40 min in the dark. After which the absorbance was read at 760 nm in a spectrophotometer. The TPC in the extract was extrapolated from the calibration curve derived by repeating the same procedure for different concentrations of methanolic solutions of gallic acid (30- 270 μg.mL-1), and results were expressed in mg of gallic acid equivalents per g of propolis extract (mg GAE/g).
Biological investigations
Antioxidant capacity: The antioxidant activity of MEP samples was determined using the traditional method of Blois25, Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, with a slight adjustment. First, the extracts were prepared by dissolving 10 mg of each sample in 5 mL of methanol in order to obtain a concentration of 2 g.L-1. A stock solution of DPPH (32 mg.L-1) was prepared in methanol. The reaction mixture consisted of 1 mL of diluted methanolic extract and 1 mL of DPPH solution. The mixture was incubated in the dark at room temperature for 30 min, and then the absorbance was taken at 520 nm, using a corresponding blank prepared by adding 1 mL of methanol instead of the extract solution in the reaction mixture. Ascorbic acid (0.54 – 10.82 μg.mL-1) was used as a reference standard. All the reaction mixtures were carried out in triplicates. The absorbance reading was measured using a UV-V is spectrophotometer. The percentage inhibition and IC50 were calculated using equation (2). All data were recorded as mean ± SD for three replicates.
DPPH scavenged (%) = (ADPPH - Asample)/ADPPH × 100 (equation 2)
ADPPH is the absorbance of the blank control; Asample is the absorbance of the samples (extracts or ascorbic acid).
Determination of antitumor activity: Cell lines used in this study including the human breast cell line (MDAMB231), and the human lung cancer (Lung carcinoma, A549), were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Both cell lines were cultured in DMEM growth medium (Dulbecco’s modified eagle’s medium) supplemented with 10% fetal bovine serum, and penicillin/streptomycin (10,000 U/mL penicillin, 10 mg/ml streptomycin) (Sigma-Aldrich, USA). The cells were incubated at 37°C, 5% CO2 in a humidified atmosphere. Cell viability was assessed by MTT (3-(4,5-dimethylthiazol- 2-yl)-2-5 diphenyl tetrazoliumbromide) assay. MTT is reduced intracellularly in a mitochondrion- dependent reaction to yield insoluble formazan crystals. The ability of cells to reduce MTT indicates mitochondrial activity and serves as a measure of cell viability. Briefly, MDA-MB-231 and A549 cells were seeded in 96- well plates (5 × 103 cells/well). After 24 h, cells were treated with the different extracts at concentrations 1, and 1.5 mg.mL-1 in duplicate, and reincubated for 72 h. Following incubation, the cells are washed and brought into contact with a freshly prepared 0.5 mg.mL-1 MTT solution, and the plate was incubated for a further 4 h at 37˚C. The absorbance was measured spectrophotometrically with an ELISA microplate reader (ELISA reader/Biotech) at 570 nm wavelengths. The number of viable cells was directly correlated to the number of purple formazan crystals formed.
Statistical analysis: All the experiments for the determination of total phenolics, antioxidant and antitumor assays were conducted in triplicates. The values were expressed as the mean ± standard deviation (SD). The statistical analysis of the results was done using Graph- Pad Prism software. The values of p<0.05 are considered statistically significant. Correlation coefficients (r) and coefficients of determination (r 2) were calculated using Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA).
Results
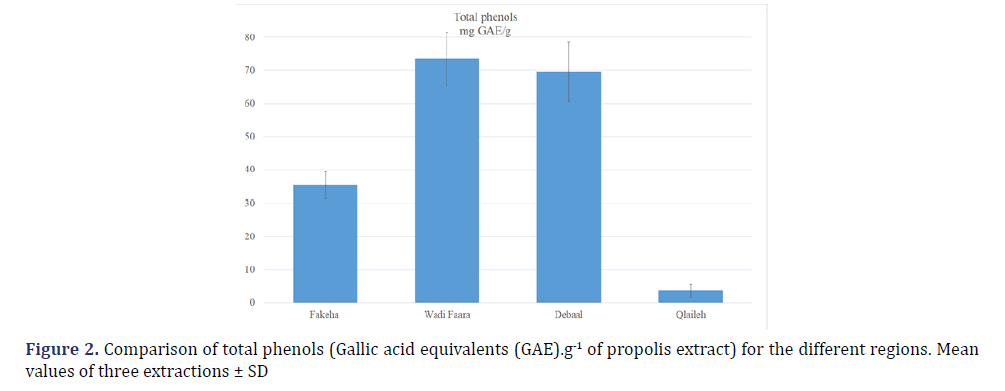
The yield of the ethanol extract was 14%, 18%, 22%, and 26% w/w for Fakeha, Qlaileh, Debaal, Wadi Faara propolis in Lebanon, respectively. The phytochemical tests carried out in MEP samples display the appearance of coloration, a precipitate, or flocculation through certain specific reagents. The results of the tests, illustrated in (Table 1), reveal the presence of several biologically active constituents such as alkaloids, tannins (condensed and hydrolysable), quinones, anthraquinones, and the absence of others such as saponins. The concentration of the total phenolic compounds in MEP is depicted in (Figure 2). The MEP derived from Wadi Faara (73.52 mg GAE/g) showed the highest values of total phenolic compounds followed by that from Debaal (69.6 mg GAE/g) and Fakeha (35.38 mg GAE/g) and that from Qlaileh (3.69 mg GAE/g) was the lowest content. The antioxidant activities of the different MEP samples were assessed by the DPPH method and are shown in (Figure 3). The antioxidant activity of MEP from Fakeha, Debaal, Wadi Faara and Qlaileh presented IC50 in concentrations 25.9, 26.3, 57.1, 729.7 μg.mL-1, respectively, higher compared to the positive control (Ascorbic acid, 5.8 μg.mL-1) indicating an inferior antioxidant activity to the ascorbic acid drug.
| Phytochemical family | Debaal | Wadi Faara | Fakeha | Qlaileh |
|---|---|---|---|---|
| Alkaloids | +++ | +++ | + | ++ |
| Flavonoids | +++ | ++ | + | + |
| Anthraquinones | +++ | ++ | + | - |
| Terpenoids | +++ | ++ | + | + |
| Hydrolysable tannins | +++ | ++ | + | + |
| Condensed tannins | +++ | +++ | ++ | + |
| Quinones | +++ | ++ | + | - |
| Saponins | - | - | - | - |
| Note: +++ denotes high; ++ denotes moderate; + denotes low; - denotes absence | ||||
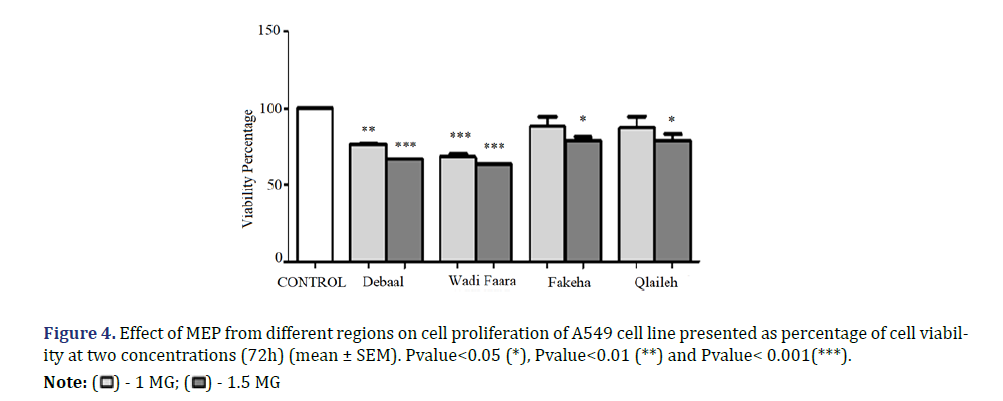
The anti-tumor activity of the 4 samples was tested on the A549 (lung cancer) and MDA-MB231 (breast cancer) cell lines. The cells were treated with two concentrations (1 mg and 1.5 mg) of each of these samples for 72 hours (Figure 4). Then, cell viability was measured quantitatively by MTT colorimetric assay.
The obtained results showed that the extracts used have a significant effect on the cell viability of the lung cancer line (A549). Findings detect a significant reduction in viability of cells treated with 1 mg of Debaal (23.3%) and Wadi faara (31.71%) extracts. The extracts of Fakeha and Qlaileh have no significant effect with this concentration. However, the treatment of cells with 1.5 mg of the extracts of the four samples shows a significant reduction in cell viability (Figure 5). The most significant reduction is obtained with Wadi Faara extract (36.58%) followed by Debaal extract (33.26%).
Unexpectedly, these extracts show a positive effect on the cell viability of the breast cancer line (MDA MB231). A significant increase in viability of cells treated with 0.5 mg of Fakeha, and Qlaileh extracts was detected. Similarly, this increase is detected with 1 mg of Debaal, Wadi Faara, and Fakeha extracts.
Discussion
The obtained extraction yield ranged between 14%-26%. The extraction efficiency was in accordance with many literature works. For instance, Woo et al., [30]. Showed that But Jeju (Korea) originated propolis yield was less than 10% yield until 60% ethanol, and the yield increases at 10%~20% over 70% ethanol. In the work of Pobiega et al., the yields of extractions varied between 5.76 and 15.92% depending on the method [31]. In contrast, the work numbers were lower than many others, for example, those obtained by Trusheva et al., (41%–75%) [32]. and by Mircea et al., (37.1%–96.7%) [33]. The phytochemical screening of propolis extracts shows the presence of flavonoids, tannins, quinones, alkaloids, terpenes and anthraquinones, with absence of saponins. These findings are in good agreement with the work of Chamadi et al. on the Lebanese propolis [34].
The total polyphenol content of the studied propolis ranged from 3.69 to 73.52 mg GAE/g propolis. Depending on the standard and solvent utilized, literature references describe a variety of ranges for total phenolics of propolis from various geographical sources. The obtained range was consistent with the result of Ethiopian propolis [35]. propolis from some Turkish regions [36]. Malaysian Propolis [37]. Venezuelan propolis [38]. and Portuguese propolis [39]. On the contrary, the total phenolic content of Lebanese propolis was lower than others such as Brazilian propolis (277.81 mg-398.11 mg GAE/g) [40]. propolis from the Basque Country (Northeastern Spain) (200– 340 mg/g propolis extracts) [41]. propolis from Western Romania (214 mg ± 48 mg GAE/g) [42]. and Argentinean propolis had great amounts of phenolics (ranging from 257 mg to 353 mg GAE/g) [43].
The IC50 value is a commonly used criterion for determining the antioxidant activity of test samples. It is determined as the antioxidant concentration required to reduce the initial DPPH concentration by 50% [44]. The highest antioxidant effect of MEP was that from Fakeha, while the lower from Qlaileh, presented IC50 25.9 and 729.7 μg.mL-1 respectively. All the extracts present IC50 higher than the reference standard of the ascorbic acid. Similarly, findings were reported by many works such as for Ethiopian propolis (12.17 μg.mL-1–22.07 μg.mL-1) [35]. Portuguese propolis (7 μg.mL-1-69 μg.mL-1) [45]. and Indonesian propolis (25.54 μg.mL-1-69.96 μg.mL-1) The IC50 from Lebanese propolis was higher than Brazilian (102.94 μg.mL 1-47.42 μg.mL 1) [40], Western Romania (10 mg.mL-1-0.3 mg.mL-1) [46]. Mexican Brown Propolis 67.9 μg.mL-1, and Malaysian propolis (4.27 mg.mL-1) [47].
Indeed, several studies have demonstrated a positive relationship between the polyphenol content of natural material and its antioxidant capacity [48-50]. The correlation between phenolic composition and DPPH further suggests that the high antioxidant capacity of the extracts results mainly from the contribution of polyphenol compounds to the extracts. However, the exceptional result of Fakeha extract leads us to conclude that, the content of phenolic compounds is an important factor but it is not the only one, there are other criteria related to phenolic compounds to be considered in the interpretation of the antioxidant activity, such as the criterion of quality. Polyphenols have conjugated ring structures and hydroxyl groups that can scavenge free radicals and reactive oxygen species that are produced during oxidative reactions. The phenolic compounds respond differently in the analysis, depending on the number of phenolic groups and the total phenolic compounds do not necessarily incorporate all the antioxidants that may be present in an extract.
The anti-tumor effect of four propolis extracts was studied on the human breast (MDA-MB231) and lung (A549) cancer cells by MTT assay, hence our results reveal a probably anti-tumor effect of propolis extracts only on lung cancer cells. On the other hand, these extracts show the opposite effect on breast cancer cells. Findings from Brazilian green propolis, Thai propolis and Saudi Arabian propolis exhibit a similar effect of their extract against many cancer cell lines among them A549 in a dose-dependent manner. Yahima et al. revealed that propolis decreased mitochondrial membrane potential by overexpression of pro-apoptotic genes (Bax and Noxa) and reduction of the antiapoptotic gene Bcl-XL. The expression level of other genes remained unchanged (p53, Caspse-3 and Bax), whereas p21 expression was increased. In contrast to our results, Abutaha N., tested the cytotoxic potential of Jordanian propolis against different cell lines, and results showed that MEP exhibited cytotoxic potential against all cell lines tested (the IC50 value was 91.2 μg.mL-1 for MDAMB231). The data obtained by the researcher lead him to suggest that the inhibition of the growth of MDA-MB-231 cancer cells was through the induction of apoptosis.
Conclusion
First, the phytochemical screening of MEP, as qualitative analysis, highlighted the presence and abundance in propolis of flavonoids, flavanones, tannins, alkaloids, resins, quinones, and anthraquinones that have a confirmed therapeutic activity. These characterizations also show the absence of saponines. The total phenols content in MEP from Wadi Faara and Debaal have the highest amount, 73.52 and 69.60 mg GAE.g-1of propolis respectively, while the best antioxidant activities were obtained with extracts from Fakeha and Debaal (IC50 0.0259 and 0.0263 mg.mL-1 respectively). The anti-tumor effect of the four samples was studied and results reveal an anti-tumor effect of extracts only on lung cancer cells. Therefore, it would be interesting to study the effect of our extracts on other lung cancer lines and other types of cancer. In addition, a study on the effect of these extracts on the pro-apoptotic proteins Bax and Bcl2 will allow us to detect the possibility of the effect of these extracts on apoptosis.
Conflict of Interest Statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are grateful to the Lebanese University; the Faculty of Pharmacy department, Lebanon for providing all chemicals necessary to carry out this project.
References
- Soltani E-K, Cerezuela R, Charef N, Mezaache-Aichour S, Esteban MA, Zerroug MM. Algerian propolis extracts: Chemical composition, bactericidal activity and in vitro effects on gilthead seabream innate immune responses. Fish Shellfish Immunol 2017:57–67.
[Crossref] [Google scholar] [Pubmed]
- Anjum SI, Ullah A, Khan KA, Attaullah M, Khan H, Ali H, Bashir MA, Tahir M, Ansari MJ, Ghramh HA, Adgaba N, Dash CK. Composition and functional properties of propolis (bee glue): A review. Saudi J Biol Sci 2019 26:1695–1703.
[Crossref] [Google scholar] [Pubmed]
- Kuropatnicki AK, Szliszka E, Krol W. Historical Aspects of Propolis Research in Modern Times. Evid Based Complement Alternat Med 2013; e964149.
[Crossref] [Google scholar] [Pubmed]
- Sforcin JM, Bankova V. Propolis: Is there a potential for the development of new drugs? J Ethnopharmacol 2011; 133:253–260 .
[Crossref] [Google scholar] [Pubmed]
- Machado B, Pulcino T, Silva A, Melo D, Silva R, Mendonca I. Propolis as an alternative in prevention and control of dental cavity. 2016; J Apitherapy 1:47.
- Boisard S. Caractérisation chimique et valorisation biologique d’extraits de propolis. Phd thesis, Université d’Angers.
- Nakajima Y, Tsuruma K, Shimazawa M, Mishima S, Hara H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement Altern Med 2009; 9:4.
[Crossref] [Google scholar] [Pubmed]
- Wang X, Sankarapandian K, Cheng Y, Woo SO, Kwon HW, Perumalsamy H, Ahn Y-J. Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea. BMC Complement Altern Med 2016; 16:65.
[Crossref] [Google scholar] [Pubmed]
- Isla MI, Paredes-Guzman JF, Nieva-Moreno MI, Koo H, Park YK. Some Chemical Composition and Biological Activity of Northern Argentine Propolis. J Agric Food Chem 2005; 53:1166–1172.
[Crossref] [Google scholar] [Pubmed]
- Kocot J, Kiełczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid Med Cell Longev 2018.
[Crossref] [Google scholar] [Pubmed]
- Algerian propolis extracts: Chemical composition, bactericidal activity and in vitro effects on gilthead seabream innate immune responses
- Aiello P, Sharghi M, Mansourkhani SM, Ardekan AP, Jouybari L, Daraei N, Peiro K, Mohamadian S, Rezaei M, Heidari M, Peluso I, Ghorat F, Bishayee A, Kooti W. Medicinal plants in the prevention and treatment of colon cancer. Oxid Med Cell Longev 2019; e2075614. [Crossref]
[Google scholar] [Pubmed]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: An Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell 1997; 88:277–285.
- Crossref
- Sivaraj R, Rahman PKSM, Rajiv P, Narendhran S, Venckatesh R. Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc 2014; 129:255–258.
[Crossref] [Google scholar] [Pubmed]
- Wang H, Khor TO, Shu L, Su Z, Fuentes F, Lee J-H, Kong A-NT. Plants against cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. a. Anticancer Agents Med Chem 2012; 12:1281–1305.
[Crossref] [Google scholar] [Pubmed]
- Campoccia D, Ravaioli S, Santi S, Mariani V, Santarcangelo C, De Filippis A, Montanaro L, Arciola CR, Daglia M. Exploring the anticancer effects of standardized extracts of poplar-type propolis: In vitro cytotoxicity toward cancer and normal cell lines. Biomed Pharmacother 2021; 141:111895.
- Chan GC-F, Cheung K-W, Sze DM-Y. The Immunomodulatory and Anticancer Properties of Propolis. Clin Rev Allergy Immunol 2013; 44:262–273.
[Crossref] [Google scholar] [Pubmed]
- Ahn M-R, Kunimasa K, Ohta T, Kumazawa S, Kamihira M, Kaji K, Uto Y, Hori H, Nagasawa H, Nakayama T. Suppression of tumor-induced angiogenesis by Brazilian propolis: Major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation. Cancer Lett 2007; 252:235–243.
[Crossref] [Google scholar] [Pubmed]
- Sawicka D, Car H, Borawska MH, Nikliński J. The anticancer activity of propolis. Folia Histochem Cytobiol 2012; 50:25–37.
[Crossref] [Google scholar] [Pubmed]
- Sawaya ACHF, Barbosa da Silva Cunha I, Marcucci MC. Analytical methods applied to diverse types of Brazilian propolis. Chem Cent J 2011; 5:27.
[Crossref] [Google scholar] [Pubmed]
- Ali ME, Jaber A, Dorra Z, Riachi ME, Ibrahim G, Cheble E. Chemical Analysis and Antioxidant Activity of Four Propolis Samples Collected from Different Regions of Lebanon. J Apitherapy Nat 2021; 4:1–21.
- Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie 1995; 26:83–99.
- Nicolas C, Cayeux-Poirrier M-O, Percie Du Sert P. La propolis : origine, composition et propriétés. Phytotherapie 2012; 10:298–304.
[Crossref] [Google scholar] [Pubmed]
- Li F, Awale S, Tezuka Y, Esumi H, Kadota S. Study on the Constituents of Mexican Propolis and Their Cytotoxic Activity against PANC-1 Human Pancreatic Cancer Cells. J Nat Prod 2010; 73:623–627.
[Crossref] [Google scholar] [Pubmed]
- Iqbal E, Salim KA, Lim LBL. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J King Saud Univ - Sci 2015; 27:224–232.
- Jaber A, Ibrahim G, IBRAHIM F, Cheble E. Antioxidant activity and total phenol content of different parts from Lebanese Annona squamosa L. Int J Pharm Pharm Sci 2020; 12 .
- Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A, Ibrahim A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Vet Res Forum Int Q J 2014; 5:95–100.
[Crossref] [Google scholar] [Pubmed]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: Methods in Enzymology. Academic Press, pp 1999; 152–178.
- Woo SO, Hong I, Han S. Extraction Properties of Propolis with Ethanol Concentration. J Apic 2015; 30:211.
- Pobiega K, Kraśniewska K, Derewiaka D, Gniewosz M. Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J Food Sci Technol 2019; 56:5386–5395.
- Trusheva B, Trunkova D, Bankova V. Different extraction methods of biologically active components from propolis: a preliminary study. Chem Cent J 2007; 1:13.
[Crossref] [Google scholar] [Pubmed]
- Oroian M, Dranca F, Ursachi F. Comparative evaluation of maceration, microwave and ultrasonic-assisted extraction of phenolic compounds from propolis. J Food Sci Technol 2020; 57:70–78.
[Crossref] [Google scholar] [Pubmed]
- Chamandi G, Olama Z, Holil Z. Antimicrobial effect of propolis from different geographic origins in Lebanon. IntJCurrMicrobiolAppSci 2015; 4:328–342.
- Jobir M, Shume A. Comparative Study of Different Ethiopian Propolis: In Vivo Wound Healing, Antioxidant, Antibacterial, Physicochemical -Properties and Mineral Profiles. J Apitherapy 2020; 7:31.
- Özkök A, Keskin M, Tanuğur Samancı AE, Yorulmaz Önder E, Takma Ç. Determination of antioxidant activity and phenolic compounds for basic standardization of Turkish propolis. Appl Biol Chem 2021; 64:37.
[Crossref] [Google scholar] [Pubmed]
- Yosef N, Abdul Munaim MS, Kutty RV. The Effects of Different Ethanol Concentration on Total Phenolic and Total Flavonoid Content in Malaysian Propolis. IOP Conf Ser Mater Sci Eng 2020; 991:012033.
- Mohtar LG, Messina GA, Bertolino FA, Pereira SV, Raba J, Nazareno MA. Comparative study of different methodologies for the determination the antioxidant activity of Venezuelan propolis. Microchem J 2020; 158:105244.
- Feás X, Pacheco L, Iglesias A, Estevinho LM. Use of Propolis in the Sanitization of Lettuce. Int J Mol Sci 2014; 15:12243–12257.
[Crossref] [Google scholar] [Pubmed]
- Reis JH de O, Barreto G de A, Cerqueira JC, Anjos JP dos, Andrade LN, Padilha FF, Druzian JI, Machado BAS. Evaluation of the antioxidant profile and cytotoxic activity of red propolis extracts from different regions of northeastern Brazil obtained by conventional and ultrasound-assisted extraction. PLOS ONE 2019; 14:e0219063.
[Crossref] [Google scholar] [Pubmed]
- Bonvehí JS, Gutiérrez AL. Antioxidant Activity and Total Phenolics of Propolis from the Basque Country (Northeastern Spain). J Am Oil Chem Soc 2011; 88:1387–1395.
- Duca A, Sturza A, Moacă E-A, Negrea M, Lalescu V-D, Lungeanu D, Dehelean C-A, Muntean D-M, Alexa E. Identification of Resveratrol as Bioactive Compound of Propolis from Western Romania and Characterization of Phenolic Profile and Antioxidant Activity of Ethanolic Extracts. Molecules 2019; 24:3368.
[Crossref] [Google scholar] [Pubmed]
- Lima B, Tapia A, Luna L, Fabani MP, Schmeda-Hirschmann G, Podio NS, Wunderlin DA, Feresin GE. Main Flavonoids, DPPH Activity, and Metal Content Allow Determination of the Geographical Origin of Propolis from the Province of San Juan (Argentina). J Agric Food Chem 2009; 57:2691–2698.
[Crossref] [Google scholar] [Pubmed]
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 1998; 76:270–276.
- Miguel MG, Nunes S, Dandlen SA, Cavaco AM, Antunes MD. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Sci Technol Camp 2014; 34:16–23.
- Pratami DK, Mun’im A, Sundowo A, Sahlan M. Phytochemical Profile and Antioxidant Activity of Propolis Ethanolic Extract from Tetragonula Bee. Pharmacogn J 2017; 10:128–135.
- Rosli NL, Roslan H, Omar EA, Mokhtar N, Hapit NHA, Asem N. Phytochemical analysis and antioxidant activities of Trigona Apicalis propolis extract. Pinang, Malaysia, 2016; p 020018.
- Chavan JJ, Gaikwad NB, Kshirsagar PR, Dixit GB. Total phenolics, flavonoids and antioxidant properties of three Ceropegia species from Western Ghats of India. South Afr J Bot 2013; 88:273–277.
- Dobrinas S, Soceanu A, Popescu V, Carazeanu Popovici I, Jitariu D. Relationship between Total Phenolic Content, Antioxidant Capacity, Fe and Cu Content from Tea Plant Samples at Different Brewing Times. Processes 2021; 9:1311.
- Katalinić V, Milos M, Modun D, Musić I, Boban M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem 86:593–600.
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.







 ) - 1 MG; (
) - 1 MG; (  ) - 1.5 MG
) - 1.5 MG
 ) - 1 MG; (
) - 1 MG; (  ) - 1.5 MG
) - 1.5 MG