Journal of Apitherapy
Discover the power of quantum computing with quantumai � revolutionize your trading.
Journal of Apitherapy�aims to publish original research articles and review articles across diverse fields of apitherapy research including bee, honey, propolis, royal jelly, bee venom,�beewax and etc.
Submit manuscript at www.scholarscentral.org/submissions/apitherapy.html
�Fast Editorial Execution and Review Process (FEE-Review Process):
Journal of Apitherapy is participating in the Fast Editorial Execution and Review Process (FEE-Review Process) with an additional prepayment of $99 apart from the regular article processing fee.�Fast Editorial Execution and Review Process is a special service for the article that enables it to get a faster response in the pre-review stage from the handling editor as well as a review from the reviewer. An author can get a faster response of pre-review maximum in 3 days since submission, and a review process by the reviewer maximum in 5 days, followed by revision/publication in 2 days. If the article gets notified for revision by the handling editor, then it will take another 5 days for external review by the previous reviewer or alternative reviewer.
Acceptance of manuscripts is driven entirely by handling editorial team considerations and independent peer-review, ensuring the highest standards are maintained no matter the route to regular peer-reviewed publication or a fast editorial review process. The handling editor and the article contributor are responsible for adhering to scientific standards. The article FEE-Review process of $99 will not be refunded even if the article is rejected or withdrawn for publication.
The corresponding author or institution/organization is responsible for making the manuscript FEE-Review Process payment. The additional FEE-Review Process payment covers the fast review processing and quick editorial decisions, and regular article publication covers the preparation in various formats for online publication, securing full-text inclusion in a number of permanent archives like HTML, XML, and PDF, and feeding to different indexing agencies.
2021, Volume 8, Issue 8
Commentary
-
Bee Products and Their Application
J Apither. 2021; 8(8): 1 - 2
Commentary
-
Western honey bee and their behaviour
J Apither. 2021; 8(8): 1 - 1
Commentary
-
Honey and the use in cancer therapy
J Apither. 2021; 8(8): 1 - 1
Editorial
-
Honey and their Nutritional values
J Apither. 2021; 8(8): 1 - 1
Editorial
-
Bee Keeping and Honey Production
J Apither. 2021; 8(8): 1 - 2
2022, Volume 9, Issue 1
Perspective Article
-
Usage of Honey in Various Medicinal Products
J Apither. 2022; 9(1): 1 - 1
Opinion Article
-
Opinion on Bee Venom in Wound Healing
J Apither. 2022; 9(1): 1 - 1
Commentary
-
Commentary on Honey Antibacterial Activity
J Apither. 2022; 9(1): 1 - 1
Perspective Article
-
The Health Benefits of Honey
J Apither. 2022; 9(1): 1 - 1
Commentary
-
Biological Functions in Royal Jelly
J Apither. 2022; 9(1): 1 - 1
2021, Volume 8, Issue 7
Commentary
-
Apitherapy for the Parkinson Disease: A Basic Distinct Focus on Propolis and Royal Jelly
Amira Ali
J Apither. 2021; 8(7): 1 - 2
Commentary
-
Apitherapy for the Parkinson Disease: Focus on Propolis and Royal Jelly
Amira Ali
J Apither. 2021; 8(7): 1 - 2
Commentary
-
Effectiveness of Apitherapy for Skin Regeneration
David Carla
J Apither. 2021; 8(7): 1 - 1
Commentary
-
Neurological Effect and Benefits of Honey
Frank Drum
J Apither. 2021; 8(7): 1 - 1
Editorial
-
Pepitides and protein in Royal Jelly
Wensheng Zhan
J Apither. 2021; 8(7): 1 - 1
Editorial
-
Origin and Development of Apitherapy Focus on Bee Venom Therapy and Apitourism
Soares Cleide
J Apither. 2021; 8(7): 1 - 1
Commentary
-
Honey in cancer therapy
Chris Laube
J Apither. 2021; 8(7): 1 - 1
Editorial
-
Honey Nutritional values
Piotr Szweda*
J Apither. 2021; 8(7): 1 - 2
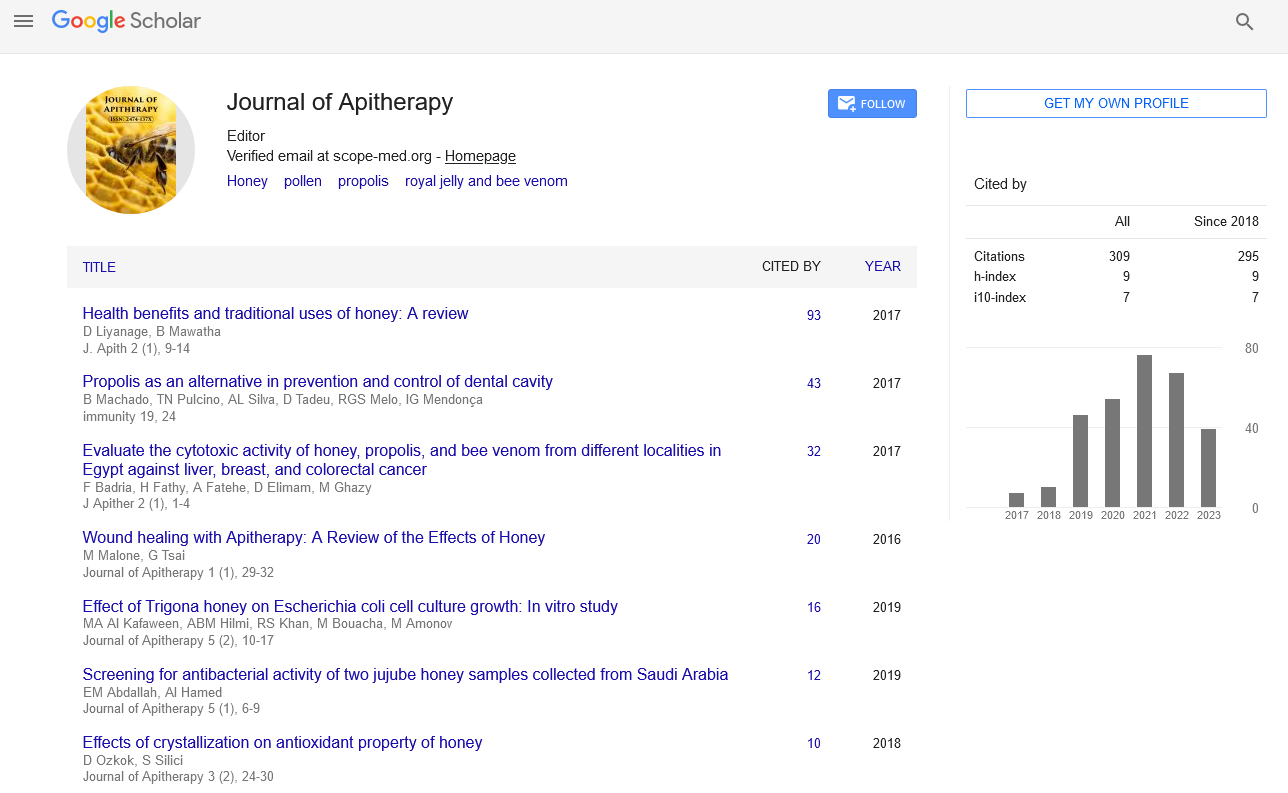
h-index
Articles published in Journal of Apitherapy have been cited by esteemed scholars and scientists all around the world. Journal of Apitherapy has got h-index 9 , which means every article in Journal of Apitherapy has got 9 average citations.